Bladder Cancer Vaccines Market to Reach US$ 1.45 Billion by 2033 at 21.32% CAGR | DataM Intelligence
Global bladder cancer vaccines market to hit US$1.45B by 2033, driven by immunotherapy advances, rising cancer burden, and expanding clinical pipelines.
Rapid advances in immuno-oncology and vaccine technologies are transforming bladder cancer treatment and accelerating global adoption.”
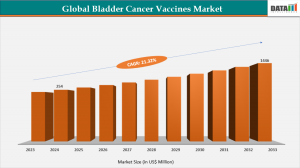
LOS ANGELES, CA, UNITED STATES, November 14, 2025 /EINPresswire.com/ -- Global bladder cancer vaccines market reached US$254 million in 2024, up from US$209 million in 2023, and is projected to reach US$1,446 million by 2033, growing at a robust CAGR of 21.32% from 2025 to 2033.— DataM Intelligence
Get a Free Sample Research PDF: https://datamintelligence.com/download-sample/bladder-cancer-vaccines-market
USA - Industry Latest News 2025:
✅ 21 Apr 2025 — Johnson & Johnson unveils TAR-200 intravesical drug-device data for early-stage bladder cancer (potential bladder-sparing therapy).
✅ 12 Aug 2025 — Merck reports KEYTRUDA (pembrolizumab) + PADCEV (enfortumab vedotin) perioperative (neoadjuvant + adjuvant) trial showed large reductions in risk of recurrence/progression and death in muscle-invasive bladder cancer (late-stage/ESMO results).
Japan - Industry Latest News 2025:
✅ 01 Oct 2025 — Astellas (Japan) announces PADCEV perioperative bladder-cancer data selected for presentation in an ESMO Presidential Symposium (significant clinical advance for MIBC).
Korea - Industry Latest News 2025:
✅ 30 Jul 2025 — Korean biotech Dx&Vx signs a $220M agreement with a U.S. firm to develop mRNA cancer vaccines (programs include mRNA approaches relevant to cancer immunotherapy research).
✅ 04 Aug 2025 — AstraZeneca’s IMFINZI receives approval/coverage notes in Korea as a perioperative immunotherapy option for muscle-invasive bladder cancer.
Europe - Industry Latest News 2025:
✅ 04 Jul 2025 — European Commission approves AstraZeneca’s IMFINZI as the first perioperative immunotherapy for muscle-invasive bladder cancer in the EU.
✅ 12 Jun 2025 — BioNTech agrees to acquire CureVac (≈$1.25B all-stock deal) to strengthen mRNA oncology capabilities — strategic M&A affecting Europe’s cancer-vaccine/oncology landscape.
✅ 18 Oct 2025 — PADCEV + KEYTRUDA perioperative results (presented at ESMO) reported to cut risk of recurrence/progression by ~60% and risk of death by ~50% — major practice-changing potential across Europe.
Market Geographical Share
North America holds a dominant share in the bladder cancer vaccines market, supported by a high prevalence of bladder cancer, strong adoption of immunotherapy, and advanced clinical research activity. The U.S. leads regional growth due to robust oncology R&D pipelines, increasing vaccine-based clinical trials, and favorable regulatory pathways for innovative cancer therapeutics.
Asia-Pacific is experiencing the fastest growth owing to rising cancer incidence, expanding healthcare access, and accelerating biotechnology development in Japan, China, South Korea, and Australia. Japan hosts substantial clinical trial activities for therapeutic cancer vaccines, while China and South Korea are investing in novel immunotherapy modalities and local manufacturing capabilities.
Get Customization in the report as per your requirements: https://datamintelligence.com/customize/bladder-cancer-vaccines-market
Key Market Drivers
✅ Rising Global Burden of Bladder Cancer
The increasing incidence of bladder cancer worldwide is driving demand for advanced therapeutic options, pushing researchers toward vaccine-based immunotherapy to improve long-term survival and reduce recurrence rates.
✅ Advancements in Immuno-Oncology Technologies
Progress in neoantigen vaccines, dendritic-cell vaccines, DNA/RNA-based platforms, and tumor-associated antigen (TAA) targeting technologies is accelerating innovation and commercialization potential.
✅ Growing Shift Toward Personalized Cancer Vaccines
Precision medicine initiatives are boosting development of patient-specific vaccines designed to boost targeted immune responses, reduce side effects, and enhance therapeutic outcomes.
✅ Increasing Clinical Trials and Research Funding
Government bodies, hospitals, and biotech developers are intensifying clinical research, resulting in an expanding pipeline of therapeutic vaccines aimed at early-stage, recurrent, and high-risk bladder cancer.
✅ Rising Adoption of Combination Therapies
Vaccines combined with immune checkpoint inhibitors, chemotherapy, or targeted therapies are showing promising efficacy, encouraging adoption among oncologists and boosting market penetration.
✅ Expanding Healthcare Investments in Emerging Markets
Improving healthcare infrastructure and rising focus on modern oncology care across Asia-Pacific, Middle East, and Latin America are strengthening vaccination-based cancer treatment accessibility.
Segments Covered in the Bladder Cancer Vaccines Market:
By Product (Bacillus Calmette-Guerin (BCG), and Others)
By Route of Administration (Intravesical, and Others)
By Distribution Channel (Hospitals, Government Organizations, and Others)
Regional Analysis for Bladder Cancer Vaccines Market:
⇥ North America (U.S., Canada, Mexico)
⇥ Europe (U.K., Italy, Germany, Russia, France, Spain, The Netherlands and Rest of Europe)
⇥ Asia-Pacific (India, Japan, China, South Korea, Australia, Indonesia Rest of Asia Pacific)
⇥ South America (Colombia, Brazil, Argentina, Rest of South America)
⇥ Middle East & Africa (Saudi Arabia, U.A.E., South Africa, Rest of Middle East & Africa)
Buy Now & Get 30% OFF — Grab 50% OFF on 2+ reports: https://www.datamintelligence.com/buy-now-page?report=bladder-cancer-vaccines-market
Major Key Players: Merck & Co., Inc., Shionogi & Co., Ltd., ImmunityBio, Inc., Altor BioScience, LLC, Hamlet BioPharma, Asieris Pharmaceuticals, Pfizer Inc.
✅ ImmunityBio, Inc. — Market leader among the four: ImmunityBio’s IL-15 agonist Anktiva® (N-803 / nogapendekin) plus BCG received major regulatory approval (U.S. FDA) for BCG-unresponsive non-muscle-invasive bladder cancer, giving ImmunityBio first-mover commercial advantage and the largest commercial upside of the named companies; exact company market share figures are not publicly broken out.
✅ Altor BioScience, LLC — Technology originator, limited direct commercial footprint: Altor originally developed ALT-803 (N-803) (the IL-15 superagonist platform), but development/commercialization efforts have been transferred/licensed (now advanced by others such as ImmunityBio); Altor itself no longer holds a leading commercial market share in vaccines vs. the approved product sponsor.
✅ Hamlet BioPharma — Small biotech with a targeted candidate: Hamlet’s Alpha1H program has demonstrated bladder-cancer activity in preclinical/early clinical work and positions the company as a niche early-stage player commercial market share is effectively minimal today until pivotal/approval stages.
✅ Asieris Pharmaceuticals — Regional / repurposing player with small footprint: Asieris is an Asia-based company pursuing bladder cancer approaches (including repurposed urinary antibiotics / early clinical presentations) and currently represents a modest regional, early-stage presence rather than a measurable share of the global bladder-vaccine market.
Unlimited Insights. One Subscription: https://www.datamintelligence.com/reports-subscription
Related Reports:
Bladder Cancer Treatment Market 2025
Bladder Scanner Market
Kailas Disale
DataM Intelligence 4market Research LLP
+1 877-441-4866
kailas@datamintelligence.com
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.